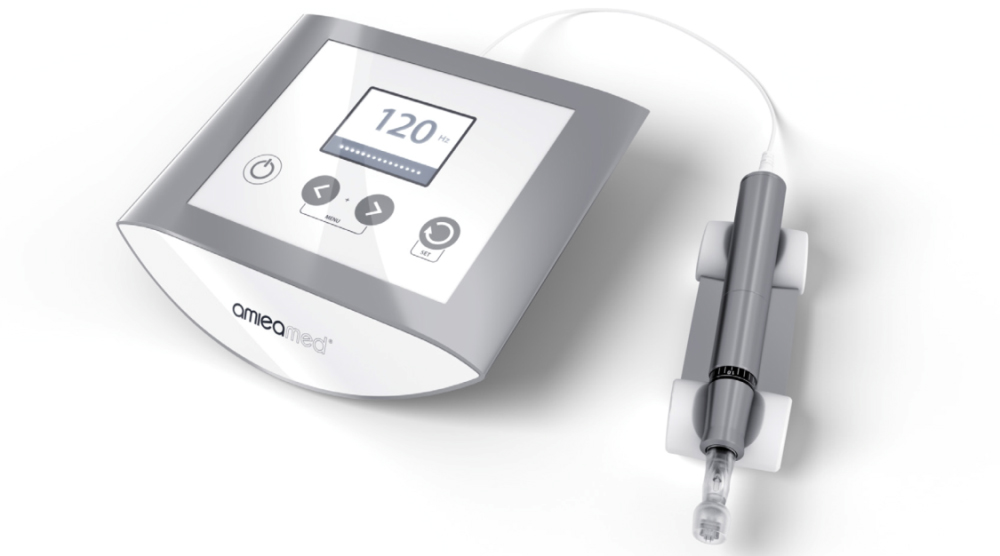
EXCEED has become the first and only FDA-cleared medical microneedling device for the treatment of both acne scars (K182407) and facial wrinkles (K180778).
Distributors of the German medical microneedling device, Derma Aesthetics, are thrilled to share the news of the second FDA clearance with the Australian market.
EXCEED by amiea med is a medical microneedling device clinically proven to treat acne scarring and now facial wrinkles, in particular, to reduce the depth of scars and to normalise the skin structure.
The procedure is designed to enhance and improve skin complexion via activation of the skin’s natural regenerative processes which stimulates collagen synthesis.
With expert precision, technique and timing, EXCEED medical microneedling triggers growth factors for building new collagen. Small, precise needles are used to penetrate the top layer of the skin vertically causing localised and controlled micro-channels without substantially affecting the structure of the epidermis.
The creation of controlled micro-channels in the skin instigates the regeneration of skin cells via a natural process of healing. Awakening the fibroblast that lies within the deeper layers of the skin, healthy collagen is naturally produced.
The gentle stimulus provided by medical microneedling is considered far more effective than many other more invasive and expensive skincare treatments. It is a quick and easy technique with little downtime. After a course of treatments, skin structure is visibly revitalised, rejuvenated, replenished and regenerated.
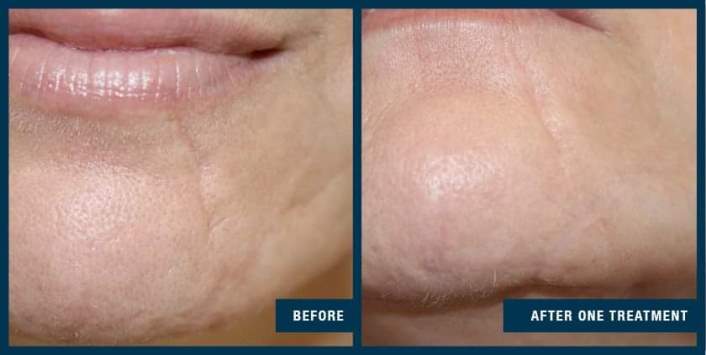
FDA clearance for treating acne scarring was supported by an extensive clinical study, which evaluated the efficacy and safety of the EXCEED medical microneedling device when used for the treatment of acne scars in subjects 18 -62 years of age.
Fifty-six subjects were treated with four microneedling sessions, 30 days apart. Subjects were assessed for changes in acne scarring using an internationally recognised acne grading scale.
The study demonstrated a statistically significant improvement in facial acne scarring, with 85 percent of subjects exhibiting a visible improvement in their facial acne scars at the end of the study. No subject’s acne scarring worsened. When questioned, 86 percent of subjects noticed a visible improvement in their acne scars compared to the start of the study, with 88 percent of subjects suggesting they would recommend the treatment to family and friends.
Glynis Ablon, MD, FAAD (Ablon Skin Institute, Manhattan Beach, CA and Assistant Clinical Professor at the University of California in Los Angeles, CA) noted:
“Previous acne scarring studies using this technology have been largely criticised for poor study design however, this study successfully reports on a suitably sized group of subjects with Fitzpatrick skintypes I-IV. In addition, the clinical data has been scrutinised as part of a 510k submission by the US Food and Drug Administration.”
“The benefit of microneedling is that the epidermis remains relatively intact and because of this, the procedure induces transforming growth factor β 3 (TGF-β3) which is associated in scarless healing rather than TGF β1 and β2 which are associated with collagen scar deposition. In addition, we found that improvements in acne scarring was not restricted to the severity of the condition or the Fitzpatrick skin type. This is really encouraging as it offers both physicians and patient’s real benefits over the traditional treatments for acne scarring.”
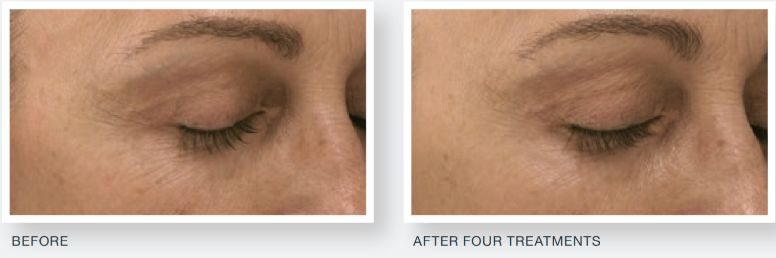
Derma Aesthetics is proud to provide aestheticians and medical practitioners with this state-of-art technology and unparalleled confidence in the treatments and results they can attain. In addition to being cleared by the US Food and Drug Administration (FDA), it is also cleared by the Australian Therapeutic Goods Administration (TGA) & Medsafe in New Zealand.
Trade in your Non-TGA/FDA Cleared Microneedling Device for EXCEED by amiea med and legitimately make medical claims and&protect your patients using the world’s first medical microneedling device with double FDA clearance.
Due to FDA regulations being tightened, we will be seeing a ripple effect in our Australasian industry ‒ we want to support your transition to medical microneedling if your current device does not have these clearances.
Simply trade in your Non-TGA/FDA Cleared Microneedling Device and you will SAVE $1168 on an EXCEED starter kit.
If your clinic is ready to focus on skin health and Corneotherapy, contact our team of experts at Derma Aesthetics on 1300 420 223 or enquiries@skincorrection.com.au.
Derma Aesthetics is also currently running these 2 exclusive offers:
– Receive a FREE, fully stocked mixing bar valued at over $2400 with every dermaviduals opening order over $4500!
– Receive a FREE premium upgrade to our complete dermaviduals mineral makeup package (A) if purchasing deco makeup package B! Only $1456.60, saving you $614.75!
These offers are valid until the 6th of September, 2019.